26+ pages a solution of kcl is saturated at 50 c 3mb. Weight of solute K C l 132 g. A how many grams of solute are disolved in 100g ofwater. KCl solubility at 20C. Check also: learning and understand more manual guide in a solution of kcl is saturated at 50 c A solution of KCl is at 50 degrees and has 50 grams of KCl dissolved in it.
A solution contains 30 g of KNO3 per 100 g of water at 20C. Click here to get an answer to your question a solution of kcl is made saturated at 353k and then its temperature changed to 100c what do you observe.
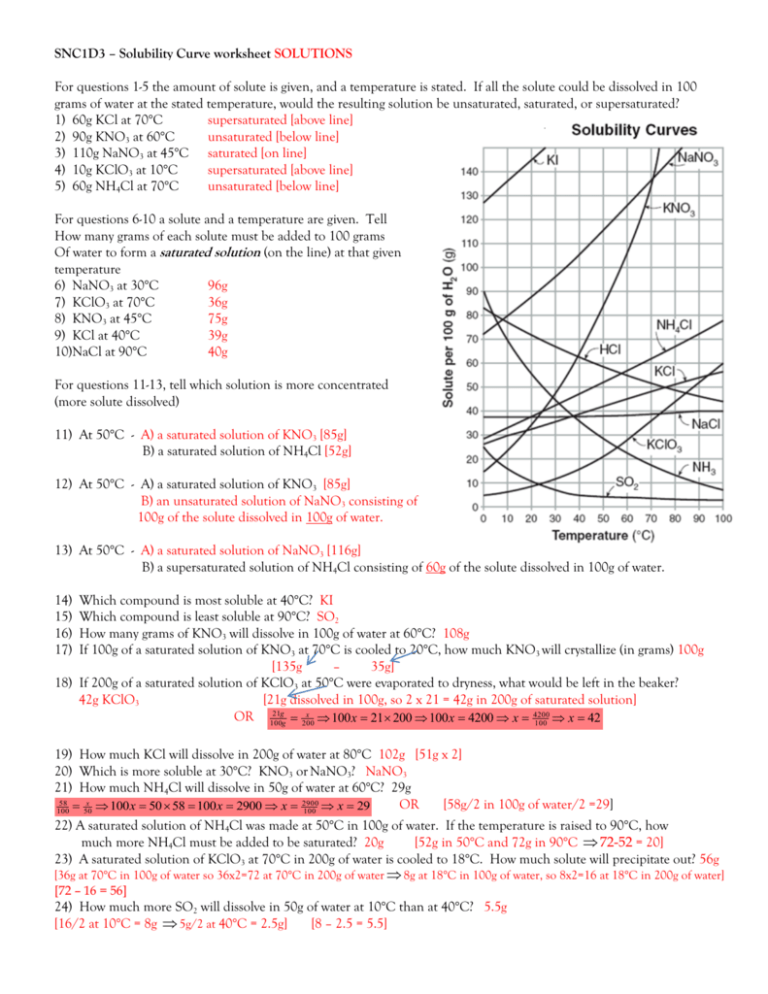
Snc1d3 Solubility Curve Worksheet Solutions For Questions 1
| Title: Snc1d3 Solubility Curve Worksheet Solutions For Questions 1 |
| Format: ePub Book |
| Number of Pages: 217 pages A Solution Of Kcl Is Saturated At 50 C |
| Publication Date: July 2020 |
| File Size: 1.2mb |
| Read Snc1d3 Solubility Curve Worksheet Solutions For Questions 1 |
 |
D If the solution is heated to 100C how much more KCL can bedissolved in the solution without adding more water.
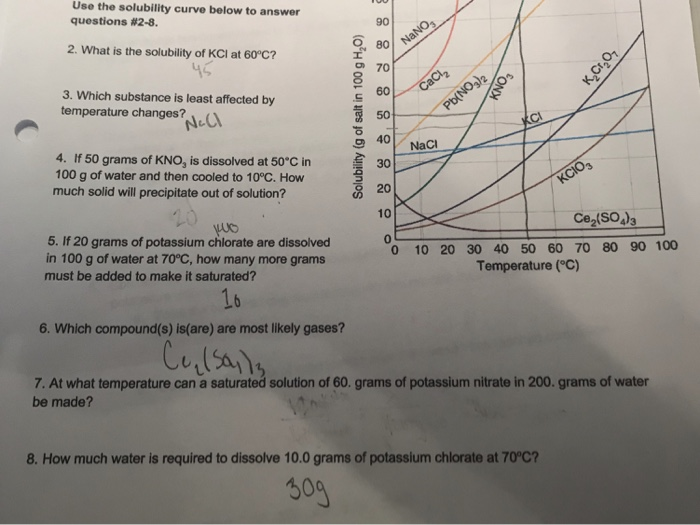
A solution in which 50 g of KClO3 is dissolved in 100 g of water at 90 degrees C is _____. This means that at 20C a saturated solution of potassium chloride will contain 34 g of dissolved salt for every 100 g of water. Ask your question. Correct answer to the question The saturation point for a solution of KCl at 50C is 40 grams100 g H2O. A solution of KCL is saturated at 50c. How much KCl should be added to the solution in order to make it saturated.




FOLLOW THE August Books Chapter AT TWITTER TO GET THE LATEST INFORMATION OR UPDATE
Follow August Books Chapter on Instagram to get the latest information or updates
Follow our Instagram